Joshua Klopper, MD
February 4, 2025
Previously we’ve discussed how cancer isn’t a single mutation event. Similarly, cancer behavior isn’t limited to cancer cell activity alone. This view is devoid of the context that surrounds and influences the cancer cells – known as the “tumor microenvironment,” or “TME.”
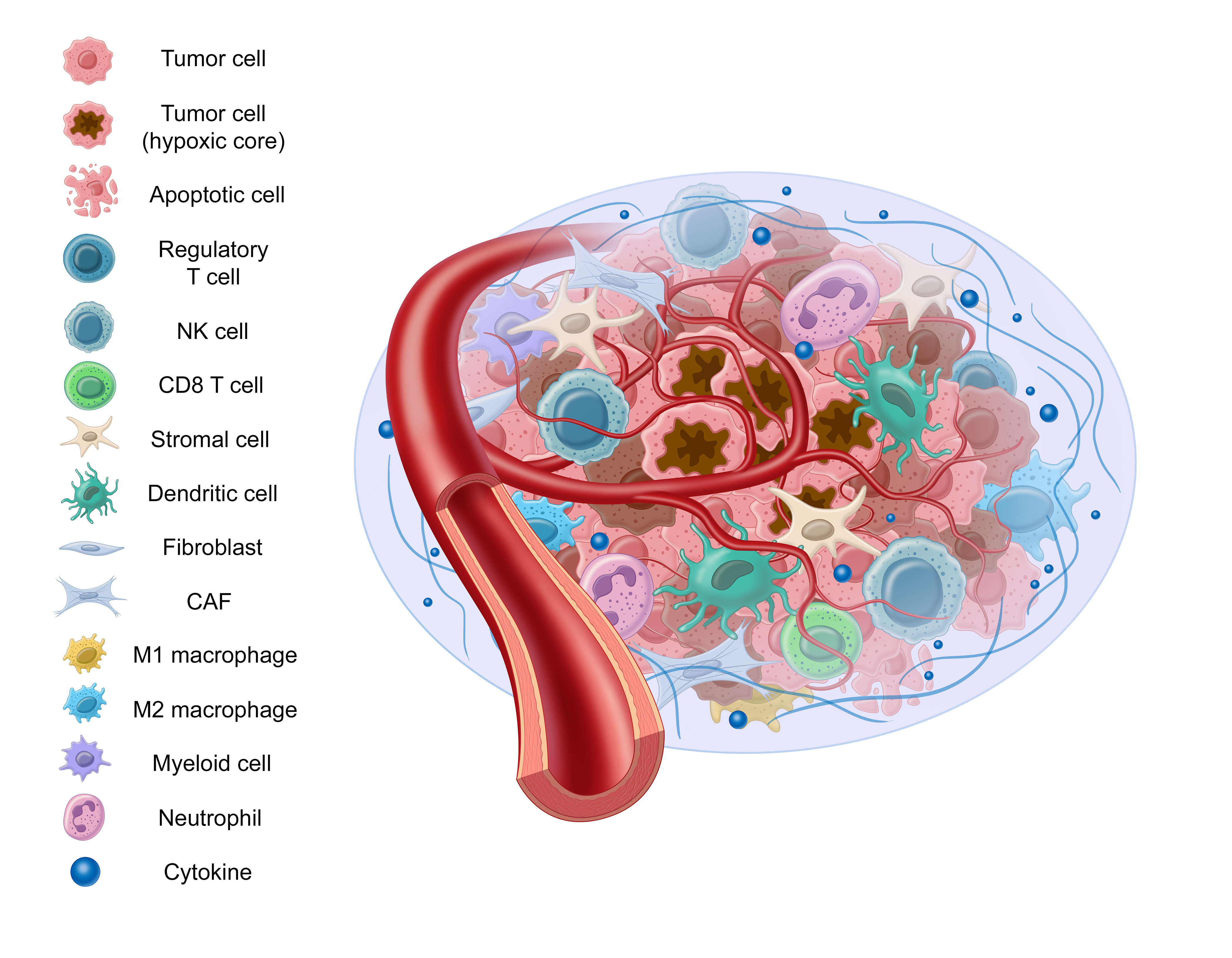
English surgeon and pathologist Stephen Paget proposed a “seed and soil” hypothesis which describes cancer cells as a seed and the tumor microenvironment as the soil. This hypothesis is now widely accepted and provides an apt analogy to help understand the role the tumor microenvironment plays in cancer progression.
There are many components to the tumor microenvironment – let’s review some of them:
Stromal tissue is comprised of supportive and connective tissue, as well as fibroblasts, myofibroblasts, vascular cells, and macrophages, among others.
Stromal tissue doesn’t act as a bystander in the development and progression of cancer. It contributes to tumor growth by secreting tumor promoting factors, promoting angiogenesis, and altering fibroblasts, all of which we’ll cover in this blog post.1
Cancer Associated Fibroblasts
In a healthy environment, these fibroblasts play an important role in healing wounds – but in the presence of transforming growth factor-beta (TGF- β), they’re converted into cancer associated fibroblasts, or “CAFs.” 2
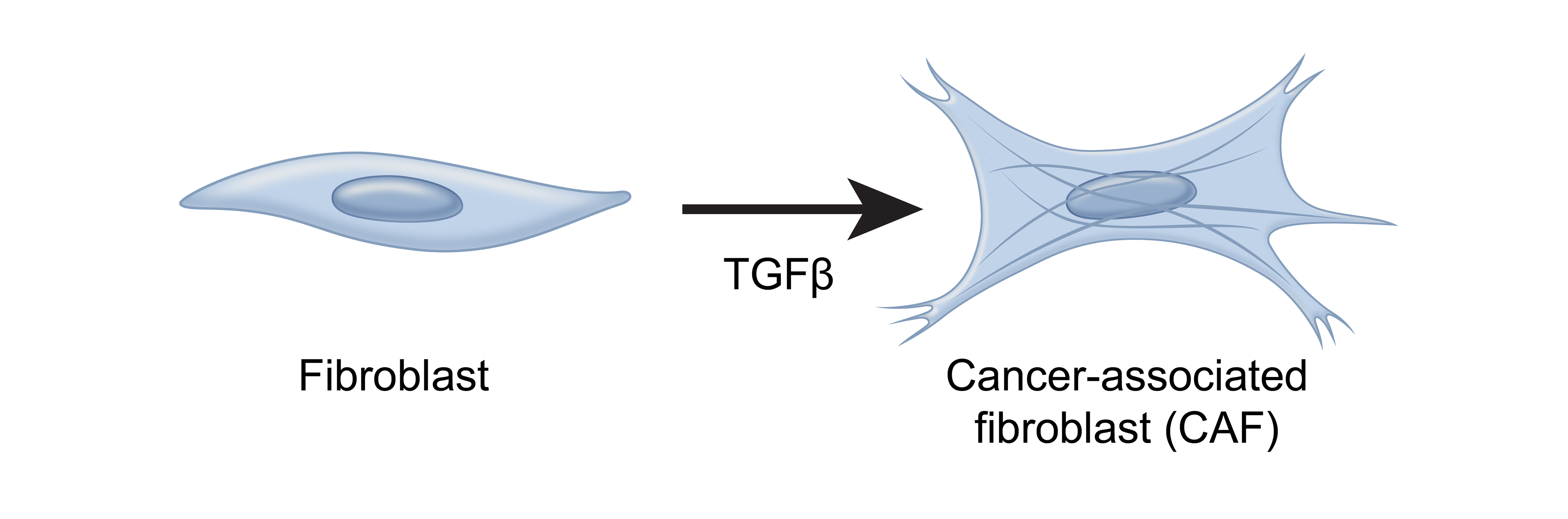
These CAFs are the most abundant cell type in the TME and have been identified as a predictor of aggressive thyroid cancer.3,4,5 Earlier this year at ENDO, research was presented regarding a novel thyroid-cancer specific CAF gene signature created using the Afirma exome-enriched molecular testing platform.5
CAFs promote tumor growth in a variety of ways, including remodeling the extracellular matrix to be more favorable to tumor growth or secreting cytokines that promote angiogenesis.3,6,7,8
Angiogenesis
Why is angiogenesis important? Blood vessels are needed to bring oxygen and nutrients to healthy, noncancerous cells, and cancer cells are no different.9 In order to grow and metastasize, new blood and lymphatic vessels must form from existing vessels. This process is known as “angiogenesis”.
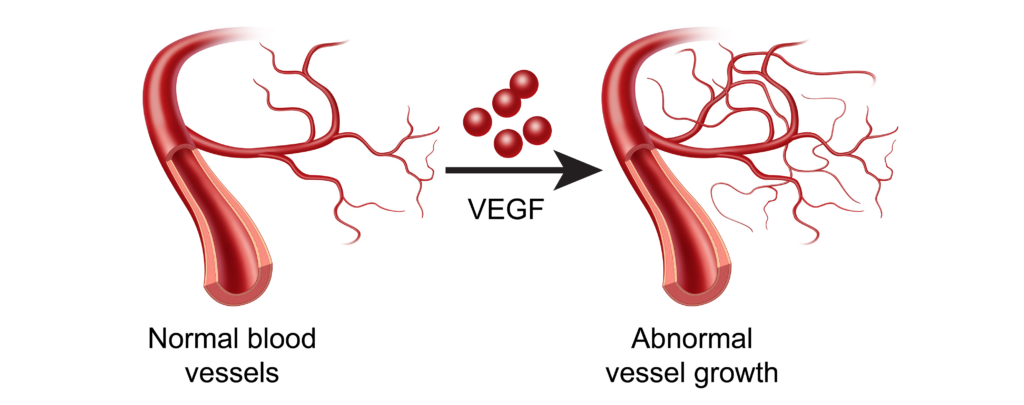
The difference in the TME is that these new vessels brought about by vascular endothelial growth factor (VEGF) or IL-8 are often too permeable, or “leaky”.10,11 Such vessels are detrimental to surrounding healthy tissue, but conducive to tumor growth.12
Hypoxia
Despite oxygen serving as a fundamental requirement for healthy cells, the tumor microenvironment is generally characterized by low levels of oxygenation, or “hypoxia.”9 Somewhere between 25 and 40% of invasive breast cancers, for instance, test positive for hypoxic markers. 9
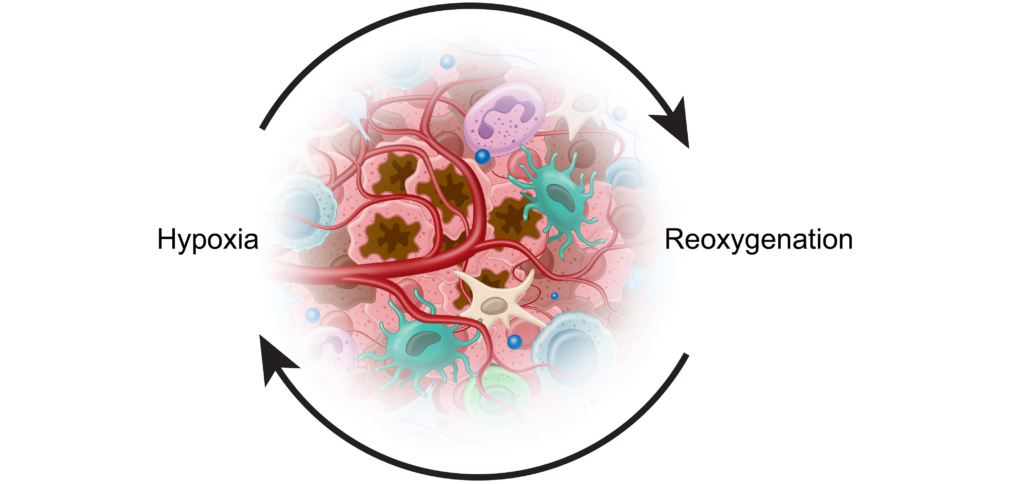
These periods of hypoxia are followed by periods of reoxygenation which lead to mutations, DNA strand breaks, mutation amplification, and other tumor-promoting complications.9,13 Cancer cells have adapted to these conditions and used them to their benefit.
The Warburg Effect
How are tumor cells able to survive and proliferate without oxygen? Healthy, normal cells use a process called Oxidative phosphorylation (OxPhos) to generate ATP, the energy for the cell. Without enough oxygen, this process can’t be completed. Cancer cells are able to switch from this process to glycolysis, which can be completed in the absence of oxygen, in order to produce energy.14,15 This is referred to as
the “Warburg Effect”.
Interestingly, it is this same phenomenon that allows tumors to be detected by PET scans.16 Glycolysis uses significantly more glucose than non-cancerous cells, so a radioactive glucose analog is injected during a PET scan, which then accumulates in cancerous areas and results in visible “hot spots” in the scan.
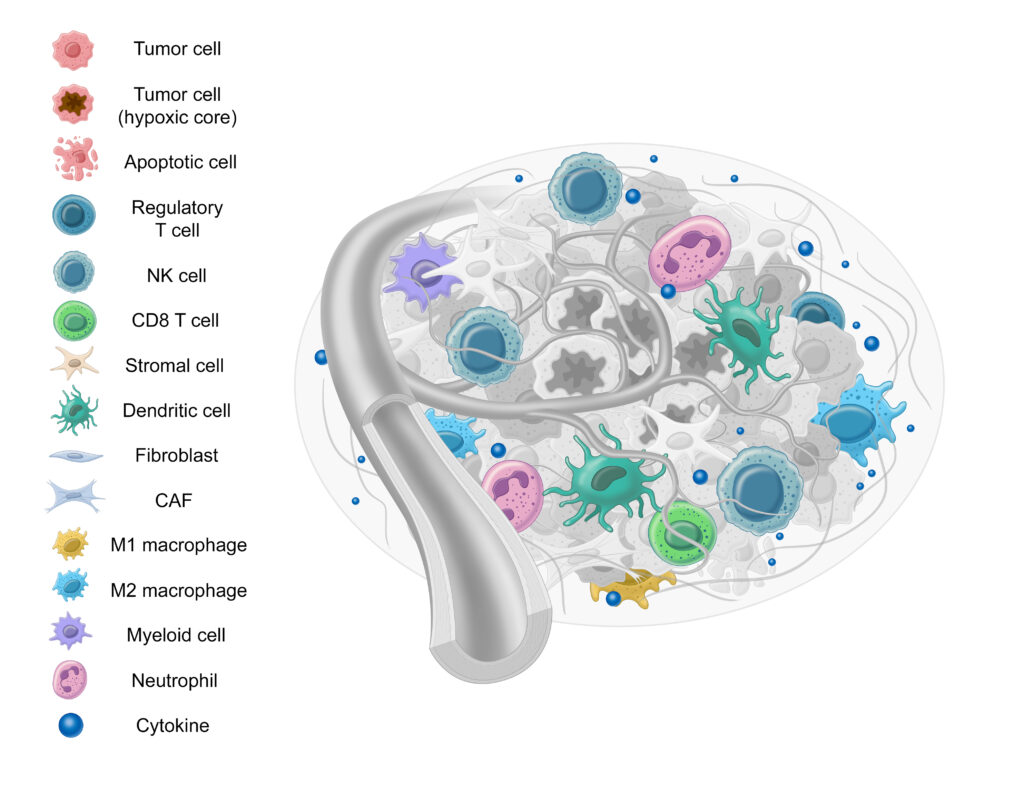
Immune cells serve a significant role in tumor progression and suppression.17-19 There are many different types of immune cells that interact with the TME, but here we’ll review the M1 and M2 macrophages, PDL1, CD4+ T cells, and CD8+ T cells.
Macrophages
First, let’s examine macrophages. Macrophages, specialized white blood cells that play a critical role in immune response, may also at times benefit the tumor microenvironment.20,21
| M1 Macrophages | M2 Macrophages |
|
M1 Macrophages, also referred to as “classical activated M1 macrophages”, are regarded as anti-tumor, as they kill tumor cells both directly and by mediating antibody-dependent cell-mediated cytotoxicity (ADCC).23,24 |
M2 Macrophages, also referred to as alternatively activated M2 macrophages, conversely, can contribute to tumor progression through immune suppression, induction of hypoxia, and angiogenic regulation, among others.23,24 |
The M2/M1 Macrophage Ratio
Due to their opposing nature, the ratio between M1 and M2 macrophages (or M1/M2 ratio) can provide potential insight into tumor progression. 23,24 While unexplored in thyroid cancer, a lower M1/M2 ratio is correlated with poor outcomes in ovarian cancer, multiple myeloma, pediatric classical Hodgkin lymphoma, colorectal cancer, and gastric cancer.25,26
PD-L1
Next, we have PDL-1. Immune cells need a way to differentiate between normal cells and cells that are dangerous to the body. This is accomplished through “checkpoint” proteins such as PD-L1. When PD-L1 on a tumor cell combines with PD-1 on an immune cell, it prevents the immune cells from killing the tumor cell.27 Tumor cells sometimes take advantage of checkpoints such as these to avoid being killed and proliferate.28,29
T Cells
T cells play a critical role in immune response to not only pathogenic infections, but cancer as well.30 There are two major types of T cells: CD8+ cells and CD4+ cells31:
| CD8+ Cells | CD4+ Cells |
|
CD8+ cells, also known as Cytotoxic T cells, have historically received more attention as a target for immunotherapy due to their ability to directly recognize and kill tumor cells (provided they carry the appropriate antigens). As a result, CD8+ cells are established as a critical biomarker associated with positive outcomes in a variety of cancers, such as breast cancer.31-33 |
CD4 cells (or Helper T Cells), on the other hand, play a more supportive role in immunity by communicating with other cells, such as CD8 cells, B cells, and macrophages to coordinate immune response.31,34,35 CD4 cells are less uniform than CD8+ cells, serving as a heterogenous group of cells that perform a variety of functions in tumor immune response, such as destroying vasculature and inducing macrophages.32 They can also contribute to tumor growth, however, by augmenting immune response in the TME.32,36,37 Their controversial role in tumor response has led to them being of great interest to researchers, especially in recent years.32 |
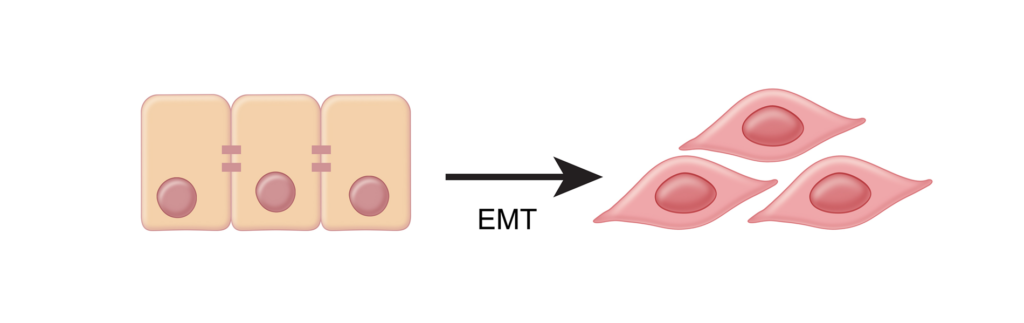
Next, we have the Epithelial to Mesencymal Transition process, or “EMT”. Similar to how TGF-β converts fibroblasts into harmful CAF’s, EMT converts epithelial cells to more motile mesenchymal cells.9 EMT is an important property of cancer cells that allows them to become invasive, metastatic, and migratory.38-39
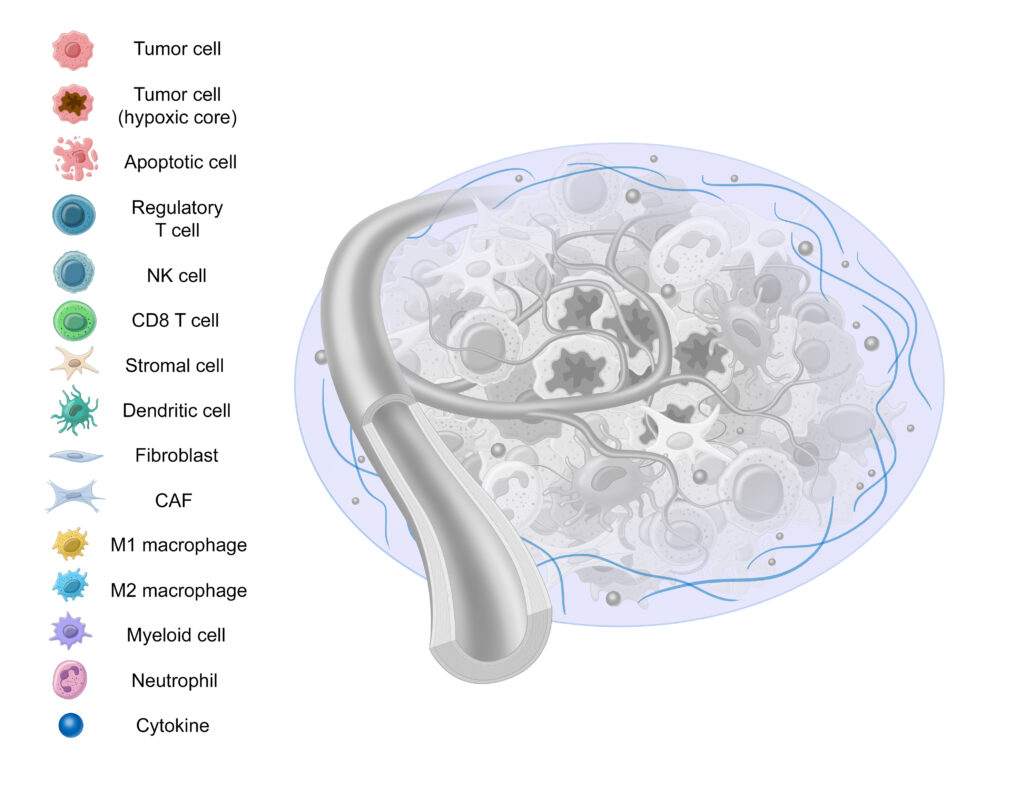
Lastly, we have the Extracellular Matrix (ECM), a highly dynamic structure present in all tissues that’s comprised of collagen, elastin, fibronectin, and other structural proteins.11,40 Serving as a form of scaffolding, the ECM serves as a physical barrier, provides structure, and helps cells attach and communicate with other cells.41,42
The ECM undergoes constant, controlled remodeling.40 Unfortunately, this same remodeling can contribute to tumor progression and resistance to therapy.43
Conclusion
Cancer development is incredibly complex, and often is not caused by a single mutation. This is why Afirma GSC uses whole-transcriptome derived analysis: it better allows us to capture the full complexity of cancer and generate a wealth of valuable information. This information not only serves as the foundation for Afirma GSC, but also creates the potential for novel research. If you’re interested in conducting research of your own or would like to learn more about Afirma GSC, fill out the form below and a genomic specialist will be in touch with you shortly.
References: