Joshua Klopper, MD
September 15, 2022
Our team recently returned from the annual Endocrine Society annual meeting (ENDO) in Atlanta, GA. It was great to see friends and colleagues in person after years of virtual meetings. We were also able to present a meta-analysis of real-world study performance of the Afirma Genomic Sequencing Classifier (GSC) and compare results to the original 2018 study validating this test (1). The Afirma GSC is run on a thyroid nodule tissue sample when the patient has an indeterminate cytology result.
Indeterminate thyroid nodules (ITNs) are clinically challenging and create stress and anxiety for patients and clinicians alike. On average, ITNs carry a cancer risk of about 25% – a risk that is too high to ignore, yet too low to feel comfortable sending all patients to surgery for confirmed diagnosis. The Afirma GSC is designed to help clinicians manage these patients.
The original Afirma GSC validation study showed:
The meta-analysis of 13 independent, single-center studies was presented at ENDO by Dr. Christian Nasr, Division Chief of Endocrinology from the University of Arizona College of Medicine. The findings from this evaluation of real-world experience with the Afirma GSC reinforce the test’s excellent performance in ruling out thyroid cancer with GSC-B results. Additionally, the real-world experience demonstrates a likelihood of malignancy in GSC-S nodules that was even higher than that reported in the original validation study. To summarize, the real-world experience with the Afirma GSC as demonstrated in the new meta-analysis shows:
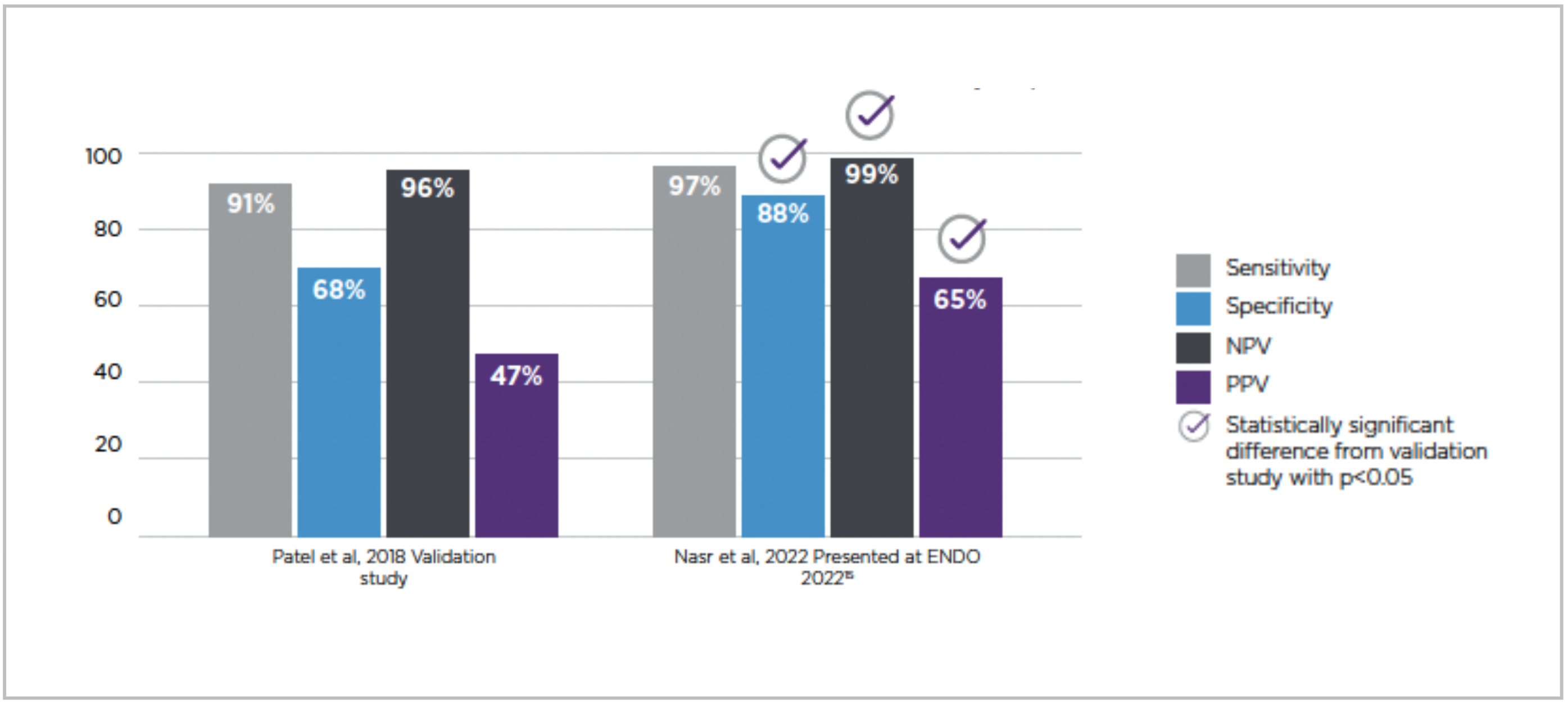
Dr. Nasr noted, “When discussing these results with colleagues and interested observers at ENDO, they were impressed that the real-world clinical performance of the Afirma GSC compared favorably to the original validation study, including the even higher risk of malignancy observed in GSC-S indeterminate thyroid nodules.”
A high-quality study (i.e., prospective, blinded, multi-center and representative of the intended test population) validating a given diagnostic test is critical to provide confidence in the test performance. Post-validation real-world studies are equally important for increasing confidence in a test’s performance and providing evidence of benefit in clinical practice outside of the controls of a validation study.
When trying to make clinical decisions for patients with a risk of cancer, many medical professionals focus on trying to confirm the cancer diagnosis. Given that most ITNs are benign, the Afirma GSC can help confidently reassure patients and providers that the risk of cancer is very low with a GSC-B result.
1 Patel KN, Angell TE, Babiarz J, Barth NM, Blevins T, Duh QY, Ghossein RA, Harrell RM, Huang J, Kennedy GC, Kim SY, Kloos RT, LiVolsi VA, Randolph GW, Sadow PM, Shanik MH, Sosa JA, Traweek ST, Walsh PS, Whitney D, Yeh MW, Ladenson PW. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg 2018; 153:817-824
2 Hu MI, Waguespack SG, Dosiou C, Ladenson PW, Livhits MJ, Wirth LJ, Sadow PM, Krane JF, Stack BC, Zafereo ME, Ali SZ, Weitzman SP, Hao Y, Babiarz JE, Kennedy GC, Kloos RT. Afirma Genomic Sequencing Classifier and Xpression Atlas Molecular Findings in Consecutive Bethesda III-VI Thyroid Nodules. J Clin Endocrinol Metab 2021; 106:2198-2207