The Afirma Team
April 23, 2025
A Meta-analysis Can Provide Data Synthesis Across Several Sites
An indeterminate thyroid nodule (ITN) carries a ~25% risk of malignancy (ROM)1, and molecular diagnostics can play a key role in ruling out cancer for the majority of ITN nodules that are benign. Molecular diagnostic tests are designed to confidently rule out malignancy in these nodules using analytical and clinical validation studies, but not all tests perform the same in a real-world setting.
Published studies of single institution experiences with a test may not be representative of the broader performance. Aggregating single-center experiences into a meta-analysis can robustly compare clinical validation (CV) metrics versus real-world performance.
Nasr et al. performed a meta-analysis of Afirma GSC, evaluating nearly 2000 ITNs across 13 sites to compare the clinical performance of Sensitivity (SN), Specificity (SP), Positive Predictive Value (PPV), and Negative Predictive Value (NPV) to the validation study2. While other tests have multiple institutional experience studies, Nasr et al. performed the meta-analysis aligning rules and assumptions to evaluate the data in a uniform method.
Afirma Performs Above Or Consistent with Validation Study Metrics
When compared to the clinical validation study, the meta-analysis found the real-world experience matched or exceeded the metrics from the CV. Notably, statistically significant improvements were seen in Specificity, PPV, NPV, and the benign call rate (BCR):
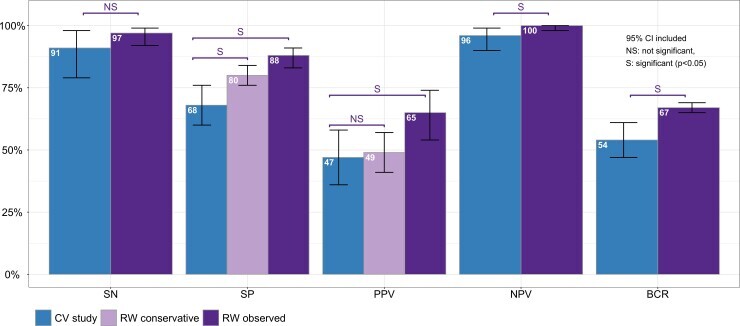
As most patients with an ITN are looking for molecular testing to guide the decision to choose surveillance over surgery, the improved Sensitivity and significant improvement in BCR demonstrate the value of an Afirma Benign result. Across all sites included in the final data set, the BCR and Sensitivity performed above or consistent with the validation study metrics. Consistent performance and high confidence in a rule-out result can be a critical component of a treatment decision, and the meta-analysis confirms the accuracy of an Afirma Benign result.
Afirma GSC utilizes a whole transcriptome-derived platform combined with machine learning. Using unique classifiers and a robust sample set for the clinical validation study, Afirma GSC’s real-world performance indicates the test continues to evolve and improve across a variety of clinical settings.
References: