The Veracyte team recently attended the 2024 American Thyroid Association (ATA) Annual Meeting in Chicago, where we were happy to connect with physicians, answer questions about Afirma, and attend several informative presentations on the latest research in thyroidology. We also had the privilege of contributing our own research to the meeting with the following three posters:
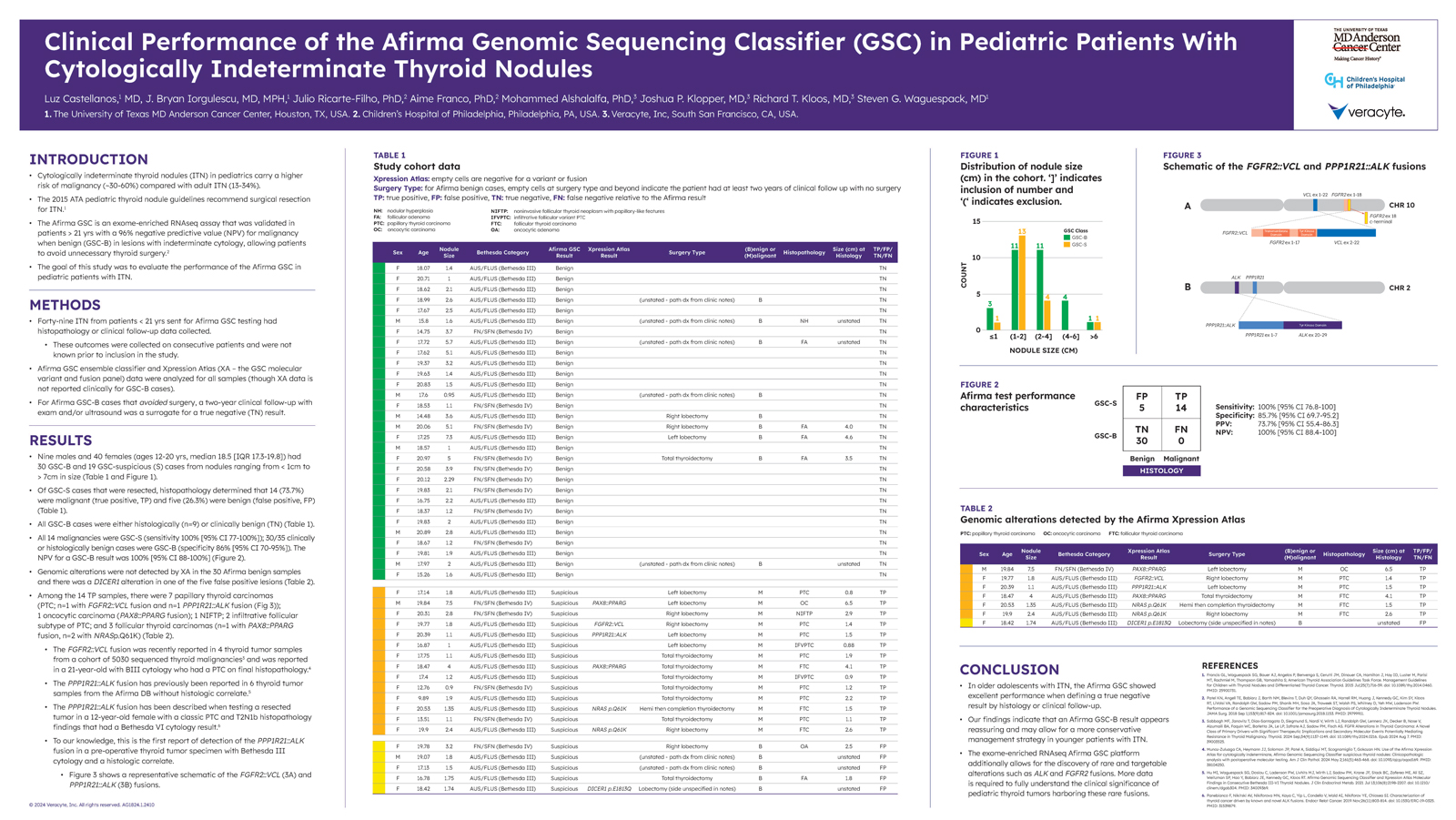
While cytologically indeterminate thyroid nodules (ITN) among adult patients carry a risk of malignancy (ROM) of 13-34%, the ROM is higher in pediatric patients, at 30-60%. Afirma GSC is validated to conclusively rule out surgery in indeterminate thyroid nodules with a 96% negative predictive value (NPV) in patients 21 years of age or older.1 This study aimed to evaluate the performance of Afirma GSC in patients under 21 years old.
The performance of Afirma GSC was measured among a cohort of 49 nodules from patients under 21 years of age that were sent for Afirma GSC testing and had either histopathology or clinical follow-up data collected. The age of the patients ranged from 12 to 20, with a median age of 18.5.
Out of the 49 nodules, 30 were found to be GSC Benign (GSC-B) – all of which were found to be histologically or clinically benign. Xpression Atlas did not identify alterations in any of these samples.
Out of the remaining 19 GSC Suspicious nodules, 5 were found to be benign and 14 were found to be malignant. 7 of these were Papillary Thyroid Carcinomas (including one with a PP1R21:ALK fusion), 3 were Follicular Thyroid Carcinomas, 2 were infiltrative follicular subtype PTCs, 1 was an oncotypic carcinoma, and 1 was an NIFTP. To our knowledge, the identified PP1R21:ALK fusion is the first found in a pre-operative thyroid tumor specimen with Bethesda III cytology and a histologic correlate.
Ultimately, this study demonstrated excellent performance of Afirma GSC among younger patients with ITN, with a 100% NPV, 73.7% PPV, 100% sensitivity, and 85.7% specificity. Additionally, the whole-transcriptome-derived analysis platform utilized by Afirma GSC allowed for the discovery of rare and targetable alterations such as ALK and FGFR2 fusions.
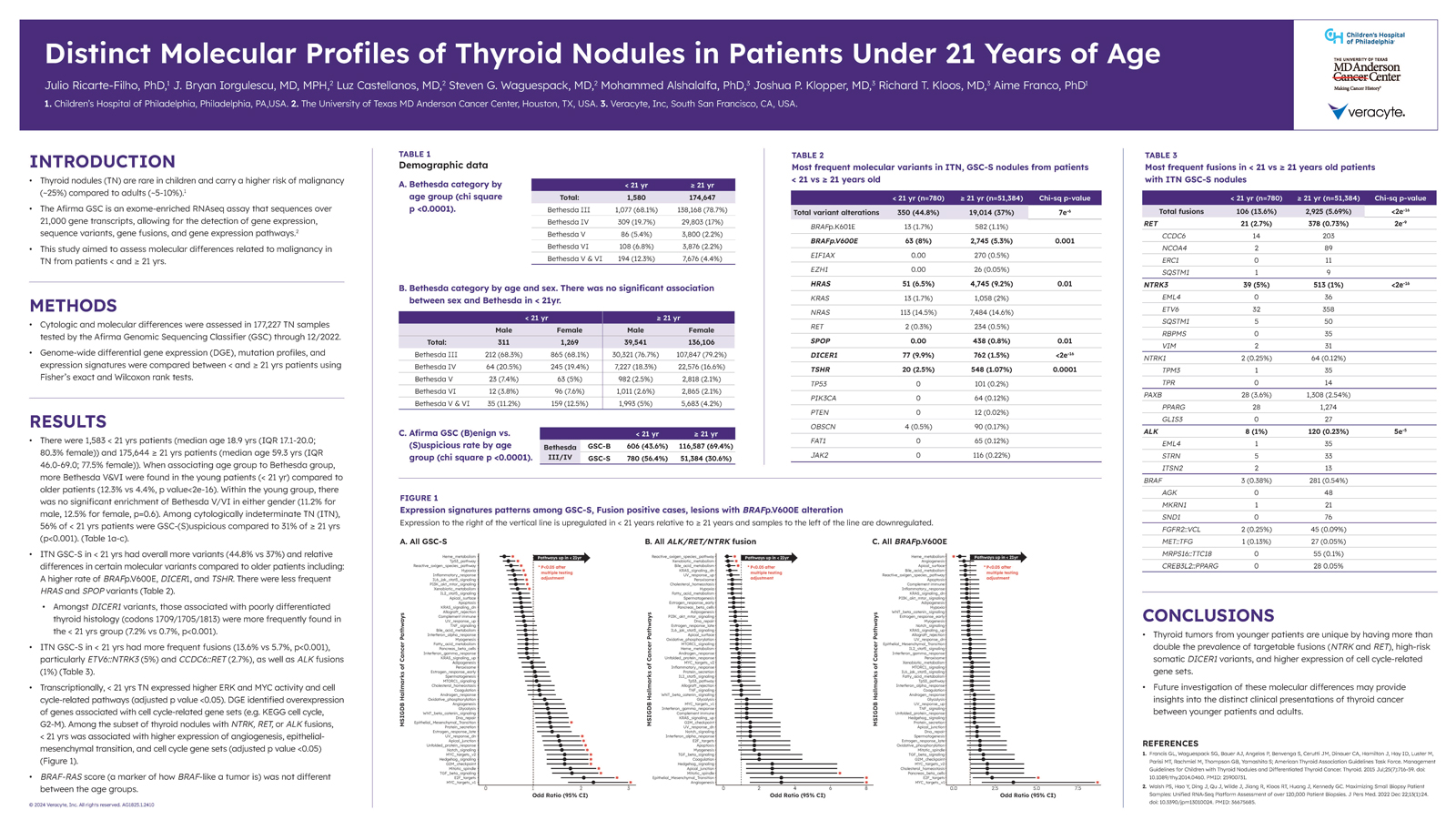
While the previous study, Clinical Performance of the Afirma Genomic Sequencing Classifier (GSC) in Pediatric Patients With Cytologically Indeterminate Thyroid Nodules, aimed to evaluate the performance of Afirma GSC in patients under 21 years of age, this study aimed to assess molecular differences in thyroid nodule samples from patients under the age of 21 versus samples from patients 21 years of age or older.
The study’s cohort consisted of a total of 177,227 Bethesda III – VI thyroid nodule samples, all tested by Afirma GSC through 12/2022:
This study found that compared to the ≥ 21 yrs patients, molecularly suspicious ITNs from patients under 21 years of age had in general more frequent variants and fusions. Higher risk and targetable fusions such as NTRK and RET were found at more than double the prevalence.
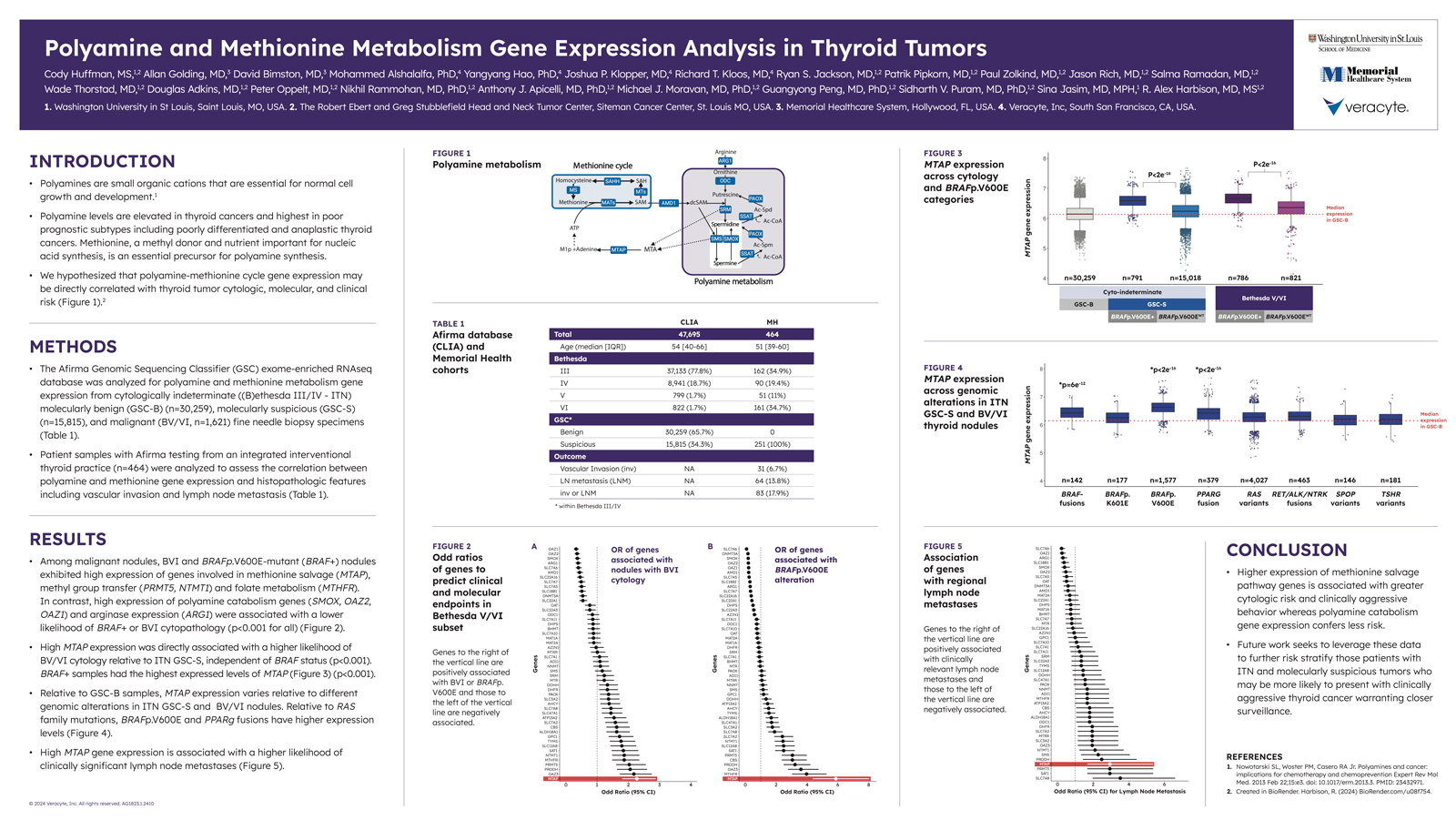
Polyamines are small molecules that play a role in DNA replication, RNA transcription, and protein synthesis.2 Along with their precursor methionine, polyamines can influence gene expression and are elevated in thyroid cancers, particularly poor prognostic subtypes such as anaplastic thyroid cancer.
This study hypothesized that there may be a direct correlation between polyamine-methionine cycle gene expression and thyroid tumor cytologic, molecular, and clinical risk. To test this hypothesis, two cohorts were analyzed for polyamine and methionine metabolism gene expression.
The two cohorts in this study consisted of:
The Afirma GSC database of 47,695 cytologically indeterminate thyroid FNA specimens – 30,259 of which were molecularly benign and the remaining 15,815 were molecularly suspicious.
464 patient samples with Afirma testing from an integrated interventional thyroid practice. Histology data from this cohort was used to assess the correlation between polyamine and methionine gene expression and histopathologic features such as vascular invasion and lymph node metastases.
This study identified a number of genes associated with a higher likelihood of Bethesda V or Bethesda VI cytology, BRAF+ cytology, or clinically significant lymph node metastases. Higher expression of methionine salvage pathway genes was found to be associated with a greater cytologic risk, and MTAP in particular was associated with a higher likelihood of BV/BVI cytology and clinically significant lymph node metastases. BRAF+ samples also had the highest express levels of MTAP. Conversely, genes associated with polyamine catabolism demonstrated less risk, though future studies are required to further risk stratify.
Conclusion
We’re happy that Veracyte could contribute to the meaningful research presented at the 2024 ATA Annual Meeting with findings made possible through our Afirma GSC and Afirma GRID. If you’d like to learn about how Afirma is leveraging whole-transcriptome-derived analysis to create the potential for novel research opportunities, fill out the form below, and someone from our team will be in touch with you shortly.