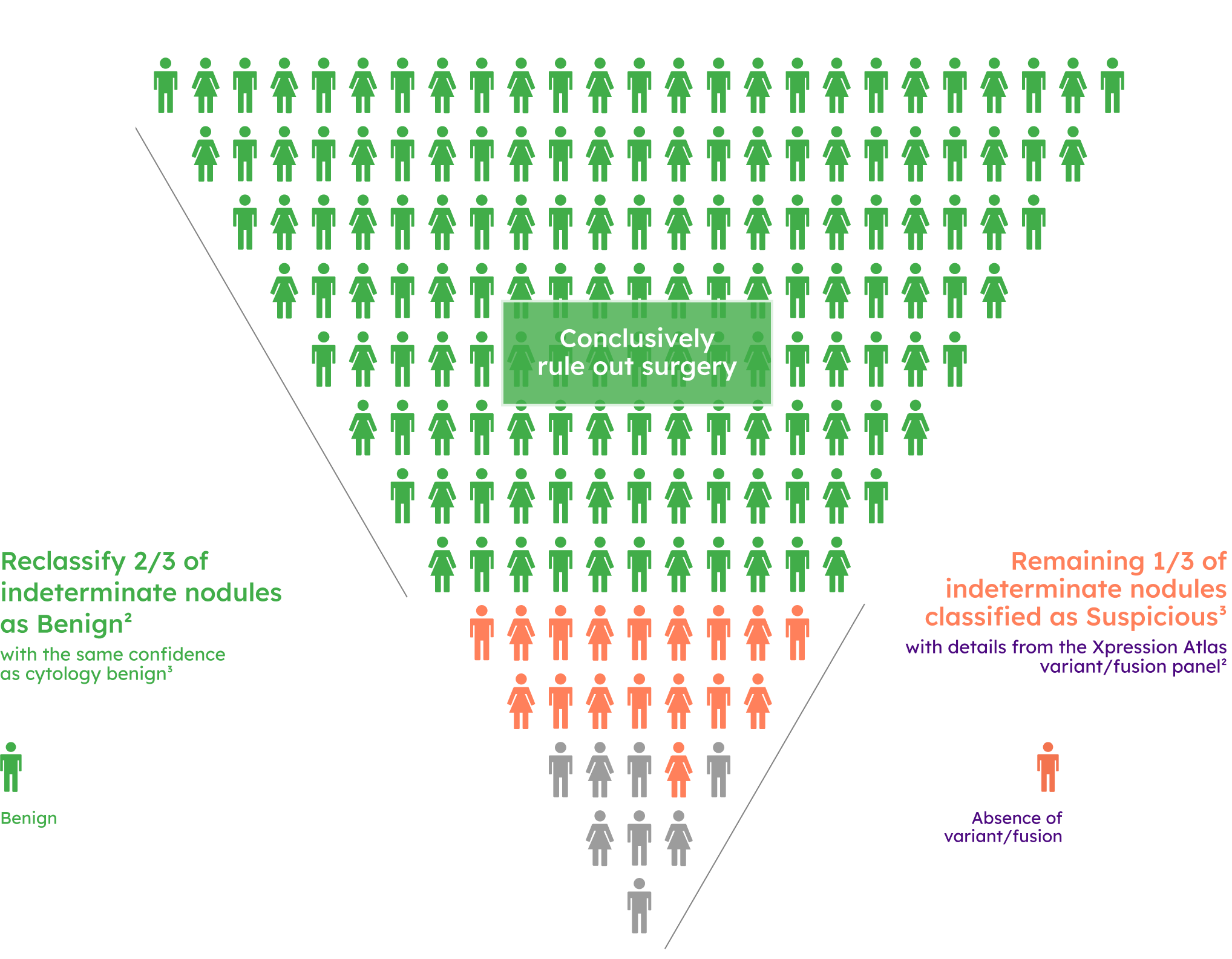
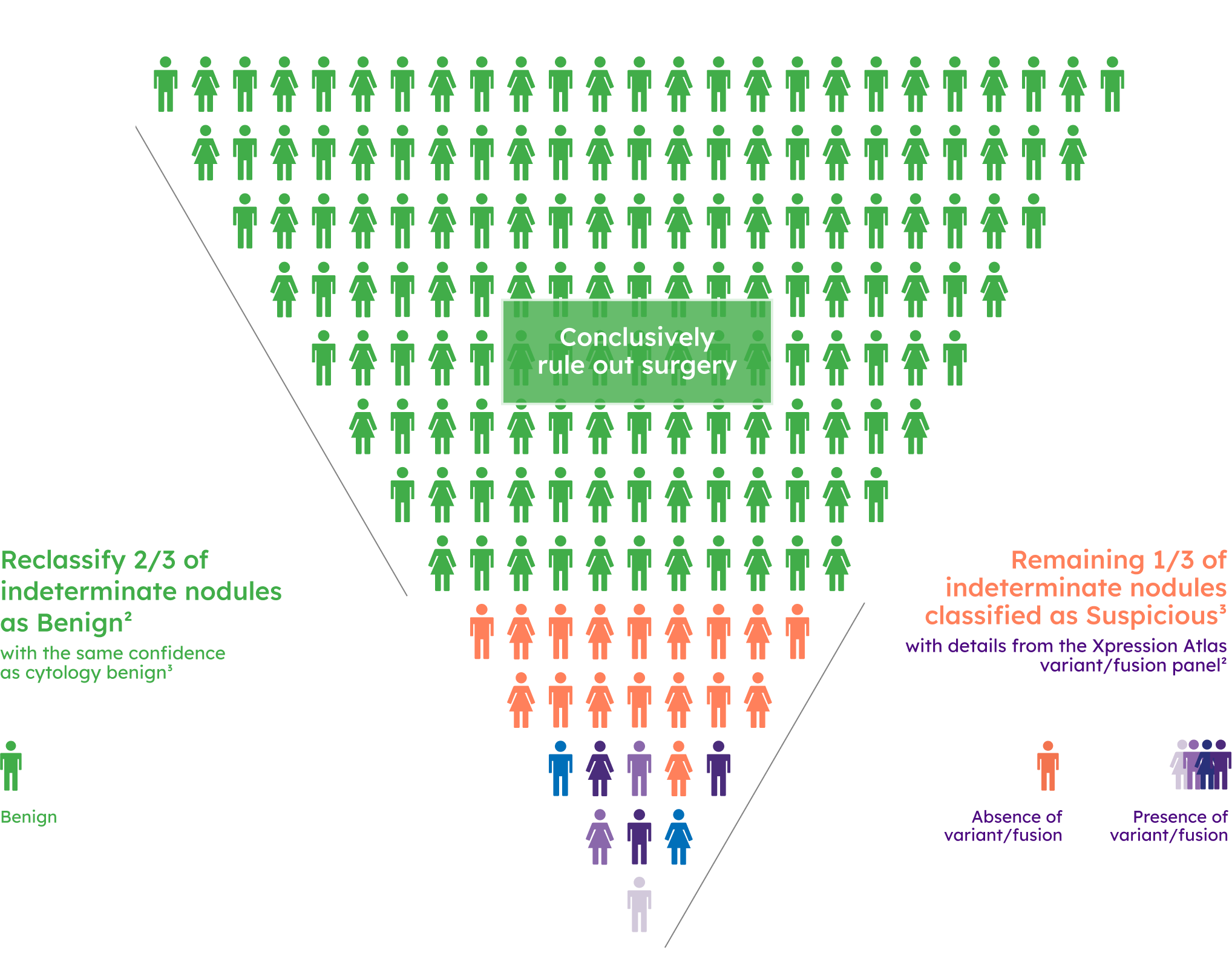
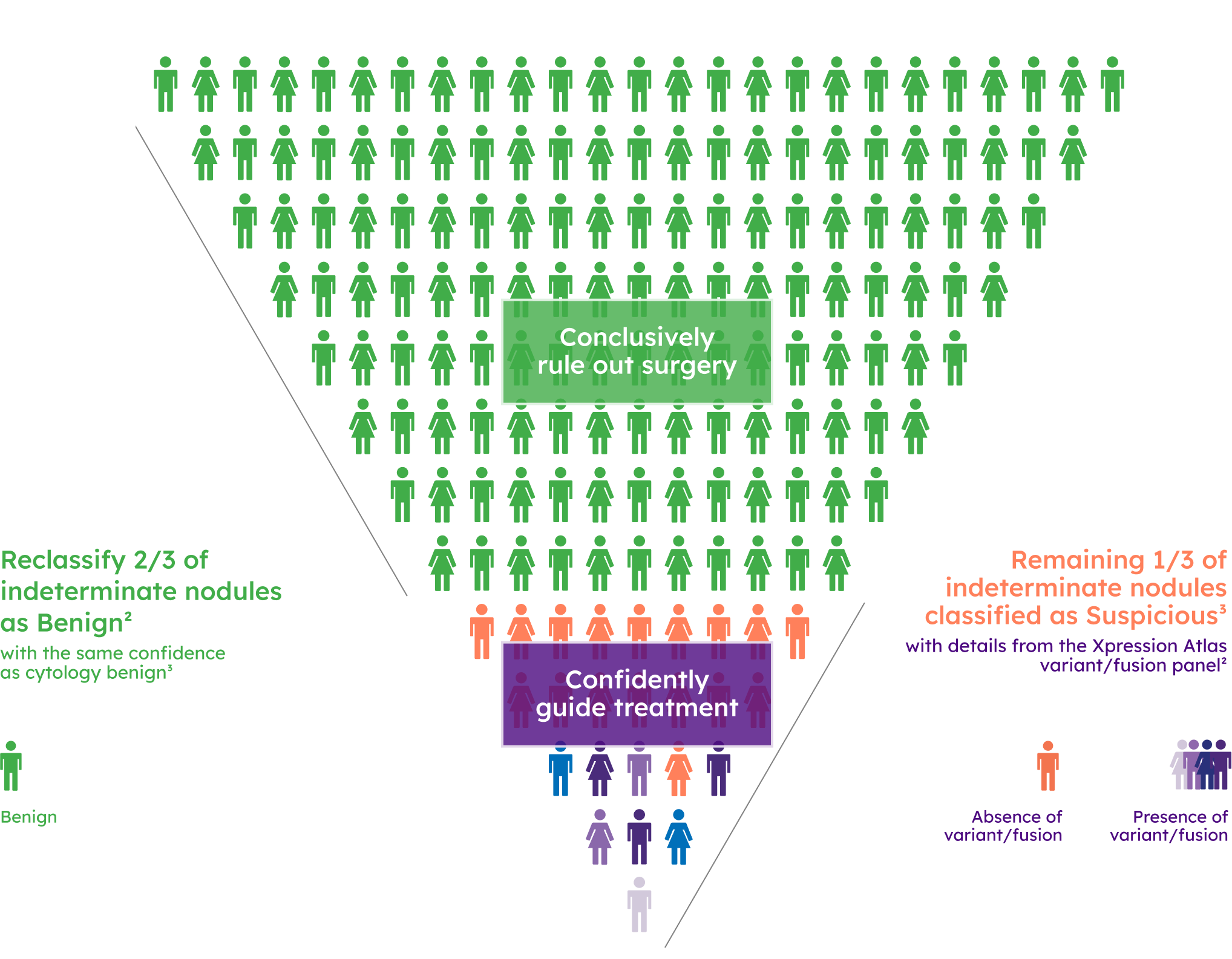
Prevalence of variant/fusion positive findings
-
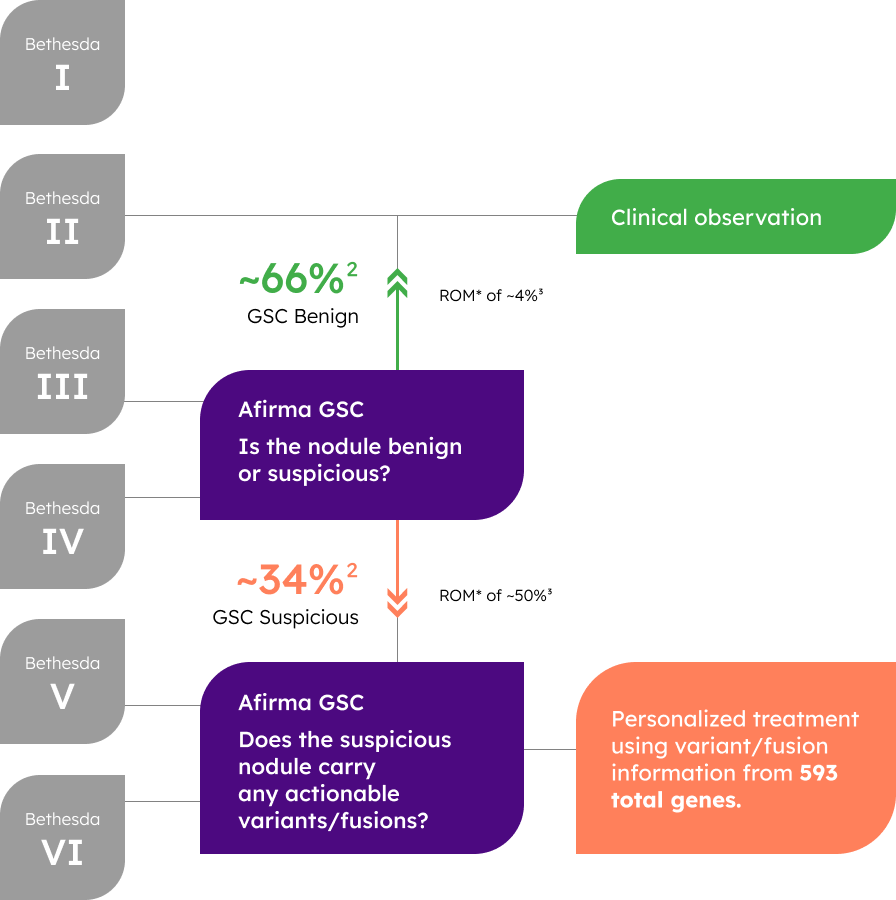
44%
of GSC Suspicious2
-
64%
of Bethesda V2
-
86%
of Bethesda VI2
- Largest thyroid gene and fusion panel available1
- Clinically validated and informed by The Cancer Genome Atlas, published literature, and Veracyte R&D discovery using nearly 40,000 samples1,14
- 593 genes, 905 variants, 235 fusions
- Expression signature data associated with variant/fusion findings are included in the report to help predict tumor behavior14,15
DNA analysis of the TERT promoter region is available for additional diagnostic and prognostic insight on the suspicious nodule