Methods: Veracyte researchers developed a test panel of 851 variants and 133 fusions in 524 genes, based on known gene alterations associated with thyroid cancer, including those identified by The Cancer Genome Atlas (TCGA) project. They then used RNA sequencing to determine the presence of these gene alterations in 76 malignant thyroid samples, representing a variety of cancer subtypes, and 75 benign samples from 88 FNA biopsies and 63 surgical tissues.
Findings: Consistent with other studies of DNA-based mutation panels, the researchers found that gene alterations appeared in only 38 of 76 (50% sensitivity) cancer samples. At the same time, 15 of 75 (20%) benign thyroid samples harbored genetic alterations (i.e. 80% specificity). See Tables 1 and 2.
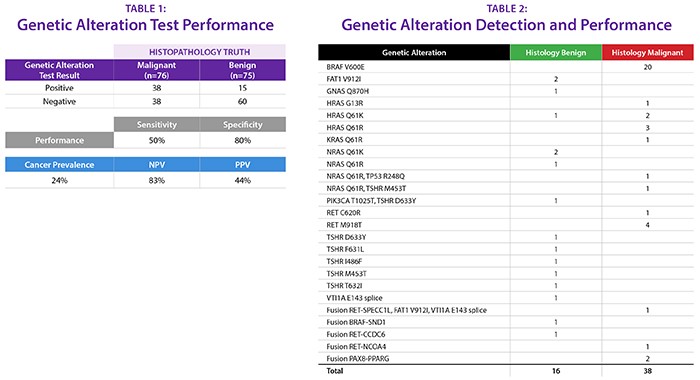
Conclusions: The researchers’ findings suggest that caution should be exercised in using gene variant and fusion panels on their own to rule out or rule in cancer in thyroid nodules. More research is needed to understand how gene variant and fusion information can best be used to guide physician decision-making in thyroid cancer diagnosis.
Link to article: http://www.endocrinologyadvisor.com/itc-2015/ata-itc-thyroid-cancer-diagnosis-genetic-variants/article/451345/