Methodology: Veracyte researchers analyzed the results of 5 next-generation sequencing (NGS) panels with increasing numbers of gene variants. The first panel included 9 point mutations and 3 gene fusions, and the remaining four panels included gene fusions and an increasing numbers of point mutations from 13 to 14 genes. The 25 fusion pairs tested could detect at least 42 unique DNA fusions.
Sensitivity and specificity were calculated for each panel to assess performance in the following sample sets:
Findings: Although sensitivity of these thyroid nodule mutation panels improved with the increasing number of gene variants, the specificity dropped dramatically. In fact, the two largest panels identified gene variants in 88-90% of FNA samples from histology-benign nodules, while the two smallest panels missed 48-58% of malignancies.
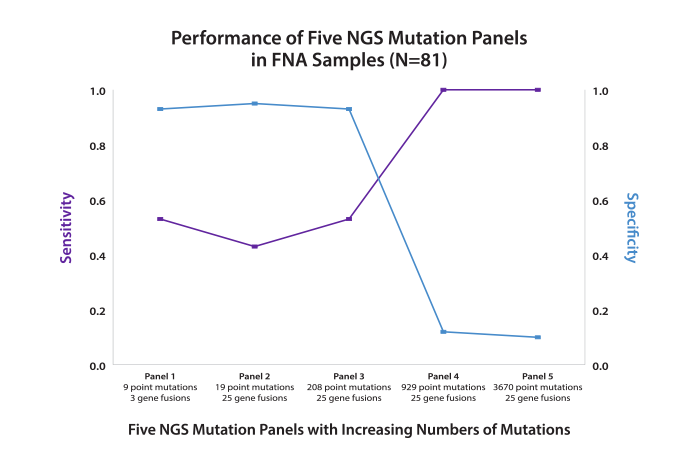
Conclusions: As more gene variants were added to thyroid nodule mutation panels, the detection of malignancy (sensitivity) increased; however, the ability to accurately detect benign nodules (specificity) declined. The drop in detection of benign nodules results from the presence of gene variants in truly benign nodules, and in a clinical setting would lead to very few patients avoiding surgery. In contrast, Afirma GEC was designed to identify benign nodules and can reduce surgeries without missing an unacceptable number of malignancies.
The full poster presented at the ENDO Society’s 97th Annual Meeting & Expo can be viewed on Afirma.com.