In that light, the authors set out to reexamine encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) through a review of cases with follow-up to (1) establish standardized diagnostic criteria and (2) identify terminology that appropriately addresses the biological and clinical characteristics of these lesions.
METHODS: Thirteen participating institutions contributed 268 patient tumor samples using the inclusion criteria for two comparison groups. Group 1 included 138 noninvasive EFVPTC without radioiodine treatment and at least 10 years follow-up. Group 2 included 130 EFVPTCs with vascular or capsular invasion and at least 1-year of follow-up. Twenty-four pathologists independently reviewed the scanned slides from the 268 tumors and the diagnoses were tabulated for the working group to review and reach consensus on clinical and pathological characteristic of tumors in the two groups.
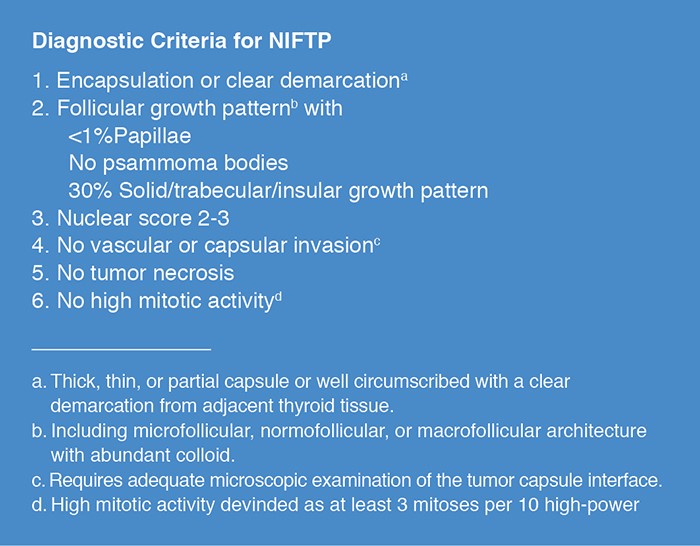
GROUP 1 RESULTS: Review of noninvasive EFVPTC cases in Group 1 lead to a consensus set of major and minor diagnostic criteria used by a majority of pathologists. Based on the criteria, 109 of 138 (79%) cases received a diagnosis of EFVPTC. The degree of expression of nuclear features of PTC correlated with the proportion of pathologists that labeled a sample EFVPTC. A subset of these cases was used to develop a simplified and reproducible set of criteria for nuclear features of PTC that can be used in the diagnosis of NIFTP. A Nuclear Score was validated ranging from 0-3 with scores of 0 to 1 being diagnostic of a benign nodule and scores ranging from 2 to 3 diagnostic for NIFTP. The final diagnostic criteria for NIFTP are detailed in Figure 1.
GROUP 2 RESULTS: Review of the invasive EFVPTC cases in Group 2 lead to a consensus set of 101 of 130 (78%) cases, including 80 (79%) with invasion of the tumor capsule, 12 (12%) with vascular invasion, and 9 (9%) with both capsular and vascular invasion.
MUTATION ANALYSIS: Mutation analysis was performed for 27 of the cases in Group 1. Of these cases 21 (78%) were mutation positive, including 8 (30%) samples positive for RAS variants.
CLINICAL OUTCOMES: Following the designation of the diagnostic criteria distinguishing Groups 1 and 2, the authors reviewed the clinical outcomes for the patients in each group. Among the 109 patients in Group 1 who were followed for 10-26 years, 61% were treated with lobectomy only, none received radioactive iodine (RAI), and all were alive with no evidence of disease. Among the 108 patients in Group 2 with 1-18 years of follow up, 78% were treated with RAI, 5 (5%) developed distant metastasis, and 2 died. Additionally, one patient had a lymph node recurrence, one had persistent disease, and 5 had detectable serum thyroglobulin that was considered an intermediate or incomplete biochemical response to therapy.
CONCLUSIONS: The authors concluded that the development of standardized criteria reflecting main morphological features, lack of invasion, clonal origin indicating the lesion is biologically a neoplasm, and a low risk of adverse outcome, a new nomenclature could be developed. Therefore, the term “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) was adopted. [1]
VERACYTE COMMENTARY: The formation of the new entity NIFTP derived from a subset of encapsulated FVPTCs is an effort to remove the malignant label from these noninvasive neoplasms. However, the authors do not go as far as labeling these lesions benign. They suggest that NIFTP is a precursor to invasive encapsulated FVPTC. Their recommendation that these lesions continue to be surgically resected is best interpreted that NIFTP be considered as a carcinoma in situ and represent an intermediary category between ‘benign with no malignant potential’, and carcinoma.
To facilitate appropriate clinical care, the current thinking remains that NIFTP tumors warrant surgical resection. Considered from this perspective, the training and categorization of NIFTP neoplasms as ‘suspicious’ by the Afirma GEC remains correct and clinically helpful.
Finally, the authors note that 22% of NIFTP neoplasms were not identified by mutational testing, which suggesting that pre-operative mutation testing may not achieve a high enough detection rate (sensitivity) of NIFTP to guide patient management.
[1] Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016;2(8):1023-9.
[2] Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15(6):e234-e242.